Introduction
Electrochemical experiments range from simple potentiostatic (chronoamperometry), to cyclic voltammetry (potentiodynamic), to complex AC techniques such as impedance spectroscopy. Moreover, each individual technique may have multiple possible experimental setups, often with a best option. This note discusses one aspect of these setups: the number of electrodes (or probes) used.
Potentiostat as a Four-Probe Instrument
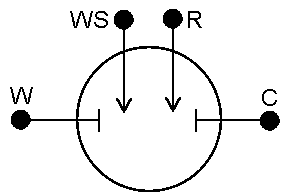
Gamry potentiostats (and some others) are all 4-probe instruments. This means that there are four relevant leads that need to be placed in any given experiment. Two of these leads—Working (green) and Counter (red)—carry the current, and the other two—Working Sense (blue) and Reference (white)—are sense leads which measure voltage (potential).
Four-probe instruments can be setup to run 2, 3, or 4 electrode measurements with just a simple change in setup. Understanding why and how to use the different modes thus is important.
Electrodes
The discussion of n-electrode mode experiments needs to address what the electrodes are. An electrode is a (semi-)conductive solid that interfaces with a(n) (electrolyte) solution. The common designations are: Working, Reference and Counter (or Auxiliary).
Working electrode is the designation for the electrode being studied. In corrosion experiments, this is probably the material that is corroding. In physical-electrochemistry experiments, this is most often an inert material—commonly gold, platinum or carbon—which passes current to other species without being affected by that current.
The Counter or Auxiliary electrode is the electrode in the cell that completes the current path. All electrochemistry experiments (with non-zero current) must have a working–counter pair. In most experiments the Counter is the current source/sink and so relatively inert materials like graphite or platinum are ideal, though not necessary. In some experiments the counter electrode is part of the study, so the material composition and setup vary accordingly.
Reference electrodes are, as their name suggests, electrodes that serve as experimental reference points. Specifically, they are a reference for the potential (sense) measurements. Reference electrodes should, therefore, hold a constant potential during testing, ideally on an absolute scale. This is accomplished by first having little or, ideally, no current flow through them, and second by being “well-poised,” which means that even if some current does flow it does not affect the potential. While many electrodes could be well-poised, there are several that are very commonly used and commercially available: silver/silver chloride, saturated calomel, mercury/mercury (mercurous) oxide, mercury/mercury sulfate, copper/copper sulfate, and more. There are other couples that are often referenced but are not typically used today, such as the normal hydrogen electrode.
Any conductive material can be used as a reference electrode, but if potential measurements are to be reported that need to be compared with other systems, use of a non-standard reference requires additional experimentation and explanation.
Four-Electrode Experiments
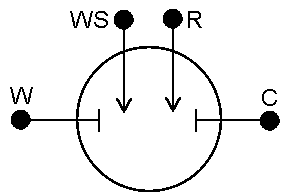
In four-electrode mode the Working Sense lead is decoupled from the working electrode, as was (and in addition to) the Reference lead.
Four-electrode setups measure potential along the B-D line in Figure 3, where there may be some “obstruction” at C. This setup is relatively uncommon in electrochemistry, though it does have its place. In 4-electrode mode, the potentials for any electrochemical reactions that are occurring at the working (and counter) electrode(s) are not being measured. What is being measured is the effect of an applied current on the solution itself or some barrier in that solution.
The most common use of this setup is to measure impedance across some solution-phase interface, such as a membrane or liquid-liquid junction. This setup can be used to make very accurate measures of solution resistance or the resistance across the surface of some material (solid-state cells).
For the full article see: https://tinyurl.com/v963w5fc